The International System of Units
When you measure something in physics, you need a standard everyone agrees on. Without that, measurements are meaningless. Over the centuries, different countries developed their own systems, and three of them became widely used:
- The CGS system (centimeter, gram, and second)
- The MKS system (meter, kilogram, and second)
- The FPS system (foot, pound, and second)
The internationally accepted system today is the Systeme Internationale d’ Unites, abbreviated as SI. You’ll see it called the International System of Units in most textbooks. SI is built on the metric system, which means conversions are based on powers of 10. That makes your calculations far simpler than working with feet and inches.
Units: Fundamental and Derived
Physical quantities fall into two categories based on how their units are defined. The units for base or fundamental quantities are called fundamental units. You cannot break these down into simpler units. The units of all other physical quantities, which you can express as combinations of fundamental units, are called derived units.
A complete set of fundamental and derived units together is called a system of units.
For any unit to be useful in science, it must satisfy four basic properties:
- It should be readily available and reproducible.
- It must be well-defined, leaving no room for ambiguity.
- It should be invariable, meaning it does not change with time, location, or conditions.
- It should be universally acceptable across all countries and scientific disciplines.
The Seven SI Base Units
The SI system rests on seven fundamental or base units. These are completely independent of each other, and you can express every other physical quantity in terms of these seven. Think of them as the building blocks of all measurement in physics.
| Base Quantity | SI Unit | Symbol | What It Measures |
|---|---|---|---|
| Length | Meter | m | Distance between two points |
| Mass | Kilogram | kg | Amount of matter in an object |
| Time | Second | s | Duration of an event |
| Electric Current | Ampere | A | Flow of electric charge |
| Temperature | Kelvin | K | Thermodynamic temperature |
| Amount of Substance | Mole | mol | Number of elementary entities (atoms, molecules, etc.) |
| Luminous Intensity | Candela | cd | Power of light emitted in a given direction |
Let’s look at each one so you understand exactly what it represents and how it’s defined.
Meter (m)
The meter is the SI unit of length. It’s defined as the distance light travels in a vacuum in exactly 1/299,792,458 of a second. This ties the meter directly to the speed of light, making it one of the most precisely defined units you’ll encounter.
Kilogram (kg)
The kilogram is the SI unit of mass. Until 2019, it was defined by a physical platinum-iridium cylinder kept in a vault in France. Now it’s defined using the Planck constant (h = 6.62607015 x 10-34 J s). This means the kilogram is no longer tied to any physical object, which makes it far more stable and reproducible.
Second (s)
The second is the SI unit of time. It’s defined as the duration of 9,192,631,770 periods of the radiation corresponding to a specific transition of the cesium-133 atom. Atomic clocks based on this definition are so accurate that they would lose less than one second over millions of years.
Ampere (A)
The ampere is the SI unit of electric current. It’s now defined by fixing the elementary charge (e) to exactly 1.602176634 x 10-19 coulombs. In simpler terms, one ampere corresponds to the flow of about 6.24 x 1018 electrons per second past a given point.
Kelvin (K)
The kelvin is the SI unit of thermodynamic temperature. It’s defined by fixing the Boltzmann constant (k) to exactly 1.380649 x 10-23 J/K. Zero kelvin (0 K) represents absolute zero, the lowest temperature theoretically possible, where all molecular motion would cease.
Mole (mol)
The mole is the SI unit for the amount of substance. One mole contains exactly 6.02214076 x 1023 elementary entities. This number is called the Avogadro constant. When you use the mole, you must always specify what entities you’re counting, whether atoms, molecules, ions, or electrons.
Candela (cd)
The candela is the SI unit of luminous intensity. It measures the power of light emitted by a source in a particular direction, weighted by the sensitivity of the human eye. It’s defined by fixing the luminous efficacy of monochromatic radiation of frequency 540 x 1012 Hz to be 683 lumens per watt.
The 2019 SI Redefinition
On 20 May 2019, the SI system underwent its most significant overhaul in decades. Four of the seven base units (kilogram, ampere, kelvin, and mole) were redefined in terms of fixed numerical values of fundamental physical constants. The kilogram was the last unit still defined by a physical artifact, and that changed with this revision.
What this means for you as a student is that every SI base unit is now anchored to an unchanging constant of nature, not to any physical object that could degrade or vary over time. The definitions became more elegant and, more importantly, more precise and universally reproducible.
Supplementary Units
Apart from the seven base units, the SI system also recognizes two supplementary quantities for measuring angles:
- Plane angle (dθ) is defined as the ratio of the length of arc (ds) to the radius (r). Its unit is the radian (rad).
- Solid angle (dΩ) is defined as the ratio of the intercepted area (dA) of a spherical surface, described about the apex O as the center, to the square of its radius (r2). Its unit is the steradian (sr).
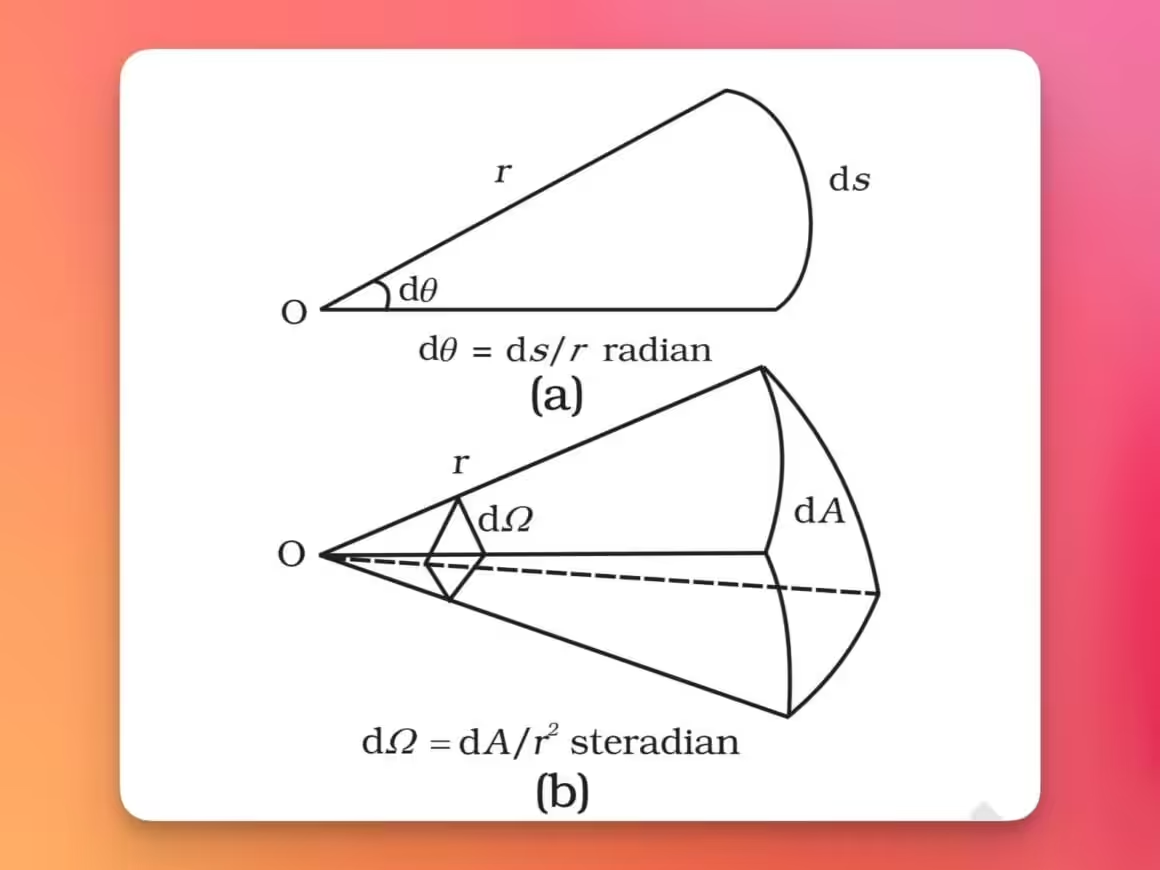
Both the radian and steradian are dimensionless quantities. They were originally classified as “supplementary units” by the SI, but since 1995 they are treated as derived units with a special status.
Derived Units
Every physical quantity that isn’t one of the seven base quantities is a derived quantity. Its unit is a derived unit, formed by combining base units through multiplication or division. For example, speed is measured in meters per second (m/s), which combines the base units for length and time.
Some derived units are used so frequently that they get their own names and symbols. You’ll encounter the newton (N) for force, the joule (J) for energy, the watt (W) for power, and the pascal (Pa) for pressure, among many others. Each of these can ultimately be broken down into combinations of the seven base units.
Whenever you express a derived unit, make sure you can trace it back to the base units. For instance, 1 N = 1 kg m s-2. This skill of dimensional analysis will help you verify equations and catch errors in your calculations.
Frequently Asked Questions
What does SI stand for and why is it important?
SI stands for Systeme Internationale d’ Unites, or the International System of Units. It is important because it provides a universal standard for measurement that scientists, engineers, and students across all countries can use consistently. Without it, comparing experimental results or collaborating across borders would be extremely difficult.
How many base units are there in the SI system?
There are seven base units in the SI system: meter (length), kilogram (mass), second (time), ampere (electric current), kelvin (temperature), mole (amount of substance), and candela (luminous intensity). All other units in physics are derived from these seven.
What changed in the 2019 SI redefinition?
In the 2019 redefinition, four base units (kilogram, ampere, kelvin, and mole) were redefined using fixed numerical values of fundamental constants of nature. The kilogram, previously defined by a physical platinum-iridium cylinder, is now defined using the Planck constant. This makes all seven SI base units independent of any physical artifact.
What is the difference between fundamental and derived units?
Fundamental (or base) units are independent units that cannot be expressed in terms of other units. Derived units are combinations of two or more fundamental units. For example, the newton is a derived unit equal to kg m/s², combining the base units for mass, length, and time.
Why is the kilogram no longer defined by a physical object?
The original kilogram prototype, a platinum-iridium cylinder stored in France, was found to have its mass drift very slightly over time due to surface contamination and material changes. By redefining the kilogram using the Planck constant, scientists ensured it would remain perfectly stable and reproducible anywhere in the world without depending on a single physical object.
What are supplementary units in the SI system?
The SI system originally classified two units as supplementary: the radian (for plane angles) and the steradian (for solid angles). Both are dimensionless quantities. Since 1995, they have been reclassified as derived units with a special status, but you will still see them referred to as supplementary units in many textbooks.
This article helped me understand the 2019 SI redefinition well enough to explain it to someone else. That is the true test of understanding.
I’ve been teaching physics for 8 years and I still find new insights in well-written resources like this. Thank you.
I’ve been teaching physics for 8 years and I still find new insights in well-written resources like this. Thank you.
I appreciate that you include both the conceptual explanation and the mathematical framework for SI units. Most resources only do one or the other.
Love how you explain the 2019 SI redefinition with real-world examples. It makes the abstract concepts much more tangible.
Reading this before my lecture made such a difference. I could actually follow along and ask better questions.
Love how you explain the 2019 SI redefinition with real-world examples. It makes the abstract concepts much more tangible.
The FAQ section answers exactly the questions I had after reading the main content. Very well thought out.
This is one of the clearest explanations of SI units I’ve found online. The way you connect the math to physical intuition really helps.
This is one of the clearest explanations of SI units I’ve found online. The way you connect the math to physical intuition really helps.
The historical context you provide for SI units makes the physics feel alive, not just equations on a page.
Reading this before my lecture made such a difference. I could actually follow along and ask better questions.
As someone self-studying physics, resources like this are invaluable. The SI units explanations are at the perfect level of detail.
As someone self-studying physics, resources like this are invaluable. The SI units explanations are at the perfect level of detail.
The mathematical formulation section is particularly well-written. You don’t skip steps, which is exactly what students need.
This article helped me understand the 2019 SI redefinition well enough to explain it to someone else. That is the true test of understanding.