Enzyme
Life is possible by virtue of the coordination of various chemical reactions that constantly take place in living organisms, such as the digestion of food, absorption of necessary molecules, and production of energy. This process involves a sequence of reactions that take place within your body under very mild conditions. All of this occurs with the help of certain biocatalysts known as enzymes.
The term enzyme was first coined by Wilhelm Kuhne in 1877, which roughly means “in yeast” in Greek (referring to the fermentation of sucrose to alcohol by zymase found in yeasts). In 1926, Sumner first crystallized the enzyme urease in pure form from jack beans (Canavalia ensiformis). For this work, he was awarded the Nobel Prize in Chemistry in 1946 along with Stanley and Northrop.
Almost all enzymes have been found to be globular proteins in nature, except two recently discovered RNA enzymes: ribozyme and ribonuclease-P. These catalytic RNA molecules, known as ribozymes, were discovered by Thomas Cech and Sidney Altman, for which they were awarded the Nobel Prize in Chemistry (1989).
Structure of an enzyme
Like any other protein, an enzyme possesses the primary, secondary, and tertiary structures. When you observe the tertiary structure of an enzyme, you’ll notice that the backbone of the protein chain folds upon itself, criss-crosses itself, and leads to the formation of many crevices or pockets.
One such pocket is the active site into which the substrate fits during a catalysis reaction. With the help of their active site, enzymes catalyse reactions at a high rate. Unlike inorganic catalysts that you may have read about in chemistry, enzymes can’t usually work efficiently at high temperatures; being proteins, they are denatured at high temperatures (on average, above 40°C).
However, special enzymes isolated from organisms that normally live under extremely high temperatures, such as hot vents and sulphur springs, are stable and retain their catalytic power even at high temperatures (around 80° to 90°C). Thermal stability is an important virtue of such enzymes isolated from thermophilic organisms, and they are extensively used in biotechnological processes.
Properties of enzymes
Enzymes have certain distinct properties that you should know about:
- Most enzymes are chemically proteins. They may possess additional inorganic or organic groups known as co-factors that are necessary for their biological activity (discussed later).
- Like inorganic catalysts, enzymes don’t actually start a chemical reaction but increase the rate of an ongoing reaction by decreasing the magnitude of the activation energy. They don’t change the equilibrium but help the equilibrium state get attained faster.
- The number of substrate molecules changed per minute by an enzyme molecule is known as the turnover number. The higher the turnover number, the higher the enzyme’s efficiency.
- Being catalysts, enzymes aren’t transformed or used up in the chemical reaction. They are released unchanged at the end of the reaction.
- Unlike inorganic catalysts, enzymes are highly specific in their action for a particular reaction and for a particular substrate. For example, the enzyme maltase acts only on the sugar maltose, not on sucrose or lactose.
How do enzymes catalyze biochemical reactions?
To understand how enzymes work, you need to first take a closer look at chemical reactions in general. Chemical compounds can potentially undergo two types of changes: physical and chemical. A physical change simply refers to a change in physical shape without involving any breaking of chemical bonds. This also includes a change in state of matter, such as ice melting or water turning into vapour.
On the other hand, when bonds are broken and new bonds are formed during the transformation, a chemical reaction is said to take place. The hydrolysis of starch into glucose is an example of an organic chemical reaction. Given below is an example of an inorganic chemical reaction.
Zn + CuSO4 → ZnSO4 + Cu
The rate of a physical or chemical process refers to the amount of product formed per unit of time. It can be expressed in the following manner:
Rate = δP/δt
If the direction happens to be specified, then the rate may also be known as the velocity. Several factors, including the temperature, influence the rates of physical and chemical processes. A general rule of thumb is that the rate gets doubled or halved for every change of 10°C in either direction.
Catalyzed reactions have been observed to proceed at much higher rates than the ones that aren’t. When you study enzyme-catalyzed reactions, you’ll find their rate is much higher than the same reaction without a catalyst.
Consider a relatively simple reaction: carbon dioxide getting dissolved in water to produce carbonic acid (H2CO3). In the absence of an enzyme, this reaction proceeds very slowly with only about 200 molecules of carbonic acid being formed every hour. However, in the presence of an enzyme named carbonic anhydrase, the reaction speeds up nearly 10 million times with about 600,000 molecules being formed every second. That’s a staggering difference, and it shows you just how powerful enzymes are.
In your body, there are thousands of different types of enzymes. Each of these enzymes catalyzes a unique biochemical reaction. A multistep chemical reaction where each of the steps is catalyzed by the same enzyme complex or different enzymes is known as a metabolic pathway.
The oxidation of glucose to pyruvic acid through ten different enzyme-catalyzed metabolic reactions is a good example of a metabolic pathway. With one or two additional reactions, this same pathway can give rise to a range of diverse metabolic end products. For example, lactic acid is formed in your skeletal muscles under anaerobic conditions while ethyl alcohol is produced in yeast cells during fermentation. Hence, different products can form in different conditions.
Mechanism of enzyme action
Now that you understand the concept of an “active site,” let’s look at how the catalytic process actually works. The chemical or metabolic conversion refers to a reaction, whereas the chemical being converted into a product is known as the substrate. Consider a hypothetical enzyme converting a substrate (S) into a product (P), in a reaction that may be depicted as:
S→P
The substrate S needs to bind to the enzyme at its active site within a particular cleft or pocket. Because the substrate has to diffuse towards the active site, there is an inevitable formation of an ES complex. Here, “E” stands for the enzyme.
This complex formation is only a transient phenomenon. When the substrate is bound to the active site of the enzyme, a new structure of the substrate known as the transition state is formed. Soon afterward, the structure of the substrate gets transformed into that of the product and is released from the active site.
The pathway of this transformation must inevitably go through this transition state structure at some point. There could be many more intermediate “altered structural states” between the stable substrate and the product, all of which are unstable.
Stability depends greatly upon the energy status of the molecule or the structure you’re dealing with. You can understand this concept better using the graph shown below:
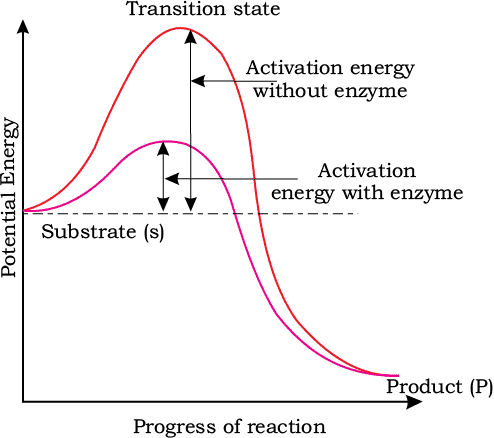
Here, the y-axis represents the potential energy content whereas the x-axis represents the progression of the reaction. Take note of two things here: firstly, the energy level difference between S and P. If P is at a lower level than S, the reaction is an exothermic reaction. In this case, you don’t need to supply energy (in the form of heat) in order to get the product.
However, in the case of both exothermic and endothermic reactions, the substrate S has to go through a much higher energy state or transition state. The difference in the average energy content of S from that of this transition state is known as the activation energy.
Enzymes work by bringing down this energy barrier and making the transition of S to P much easier. The ES complex is short-lived and soon dissociates into its product(s) P and the unaltered enzyme. The formation of the ES complex is absolutely essential for catalysis.
Models for the mode of enzyme action
Scientists have put forward three different models to explain the mode of enzyme action. Let’s walk through each one.
Lock and key hypothesis
It was proposed by Emil Fischer in 1894. According to this model, the enzyme has a rigid structure or conformation. The substrate fits into the active site of the enzyme just like a key only fits into the proper lock. The active site contains special groups having -NH2, -COOH, and -SH moieties to establish contact with substrate molecules.
After the substrate molecules come in contact with the active site of the enzyme, they undergo chemical changes and form products. Because the product no longer fits into the active site, it is released to the surrounding medium and leaves the active site free to receive more substrate molecules. Essentially, the hypothesis states that the active site of an enzyme is a rigid and pre-shaped template where only a specific substrate can bind.
This model demonstrates how a small concentration of an enzyme can act upon a large amount of the substrate. It explains why the enzyme remains unaffected at the end of the reaction. It also explains how competitive inhibitors possessing a structure similar to the substrate inhibit the enzyme.
However, the lock and key model doesn’t adequately explain the flexible nature of enzymes. It fails to explain many features of enzymatic reactions such as the effect of allosteric modulators.
Induced fit hypothesis
In 1958, Koshland proposed a more acceptable and realistic model for enzyme-substrate complex formation. According to this hypothesis, the active site of the enzyme doesn’t initially exist in a shape complementary to the substrate. Rather, it is induced to assume the said shape when the substrate is bound to the enzyme.
Think of it this way: the active site is induced to assume a complementary shape much like a hand induces a change in the shape of a glove. The model asserts that enzymes or their active sites are flexible in nature. The active site of the enzyme possesses two groups:
- A buttressing group for supporting the substrate.
- A catalytic group for catalysing the reaction.
When the substrate comes in contact with the buttressing group, the active site becomes modified to bring the catalytic group opposite to the substrate bonds, which are then broken.
The induced fit model is supported by strong experimental evidence from X-ray diffraction studies. It also explains the action of allosteric modulators and competitive inhibitors on enzymes.
Substrate strain theory
In the substrate strain theory, the substrate is strained due to the induced conformation change in the enzyme. It is also possible that when a substrate binds to the preformed active site, the enzyme induces a strain on the substrate that leads to the formation of the product.
Some scientists feel that a combination of the induced fit model with the substrate strain is operative in the mechanism of enzymatic action.
Catalytic cycle of enzyme action
You can describe the catalytic cycle of enzyme action in the following steps:
- First, the substrate binds to and fits into the active site of the enzyme.
- The binding of the substrate stimulates the enzyme to change its shape and fit more tightly around the substrate.
- The active site of the enzyme is now in close proximity to the substrate and breaks the chemical bonds of the substrate to form the new enzyme-product complex.
- Finally, the enzyme releases the products of the reaction. The free enzyme is then ready to bind to another molecule of the substrate and run through the catalytic cycle once again.
Factors affecting enzyme activity
When you think about enzyme activity, keep in mind that most enzymes are chemically proteins. Remember that the tertiary structure of a protein is vital for its biological functions. With this concept in mind, it’s easy to see why the activity of an enzyme may be affected by a change in the conditions that can disrupt the tertiary structure of its protein chains.
These conditions include temperature, pH, change in substrate concentration, or the binding of certain chemicals that regulate its activity. Let’s understand more about these factors in detail.
Temperature and pH
Enzymes usually function in a narrow range of temperature and pH. Scientists have discovered that every enzyme demonstrates its highest level of activity at a particular temperature and pH known as the optimum temperature and optimum pH. The enzyme activity steadily declines both below and above the optimum value.
At low temperatures, the enzyme is preserved in a temporarily inactive state and regains its lost activity when you raise the temperature back to normal. On the other hand, high temperature destroys enzymatic activity completely because the enzyme, being a protein, gets denatured by heat. Once the enzyme protein gets denatured, it remains inactive even if the temperature is brought down later. This is an irreversible change.
Similarly, a rise or fall in pH above or below the optimum reduces enzyme activity. Some enzymes act best in an acidic medium and others do so in an alkaline medium. For instance, pepsin in your stomach works optimally at a pH of about 2, while trypsin in the small intestine prefers a pH close to 8.
Concentration of substrate
With an increase in substrate concentration, the velocity of the enzymatic reaction rises at first. The reaction ultimately attains a peak value of velocity (Vmax) which is not exceeded by any further rise in the concentration of the substrate. The reason behind this phenomenon is that the enzyme molecules are fewer in number than the substrate molecules. After saturation of these molecules, there are no free enzyme molecules left to bind with the additional substrate molecules.
Km or the Michaelis constant is a mathematical derivation or constant that indicates the substrate concentration at which the chemical reaction catalyzed by an enzyme attains half its maximum velocity. In other words, it tells you the concentration of substrate at which the enzyme is working at half of its Vmax. A low Km means the enzyme has a high affinity for its substrate, while a high Km indicates lower affinity.
Inhibition of enzyme activity
Any substance that can bring down the velocity of an enzyme-catalyzed reaction is known as an inhibitor. Reversible inhibitors bind to enzymes via non-covalent bonds. Subsequent dilution of the enzyme-inhibitor complex leads to the dissociation of the reversibly-bound inhibitor and the enzyme regains its activity.
On the other hand, irreversible inhibition takes place when an inhibited enzyme fails to regain its activity upon dilution of the enzyme-inhibitor complex. Certain irreversible inhibitors act by forming covalent bonds with specific groups of enzymes. For example, a class of insecticides known as organophosphates produces neurotoxic effects by irreversibly binding to the catalytic site of the enzyme acetylcholinesterase.
Let’s now understand the concepts of reversible, irreversible, and allosteric inhibition in detail.
Reversible inhibition
In reversible inhibition, the inhibitor binds non-covalently to the enzyme. Furthermore, the enzyme inhibition can be reversed if the inhibitor is removed. Reversible inhibition is further sub-divided into the following categories:
- Competitive inhibition
- Non-competitive inhibition
Competitive inhibition
In this type of inhibition, the inhibitor closely resembles the real substrate structurally and is regarded as a substrate analogue. It binds reversibly to the same site on the enzyme that the substrate normally occupies by competing with it for the same. You can reverse the effect of a competitive inhibitor by increasing the concentration of the substrate [S].
There is no effect on Vmax during competitive inhibition; at a sufficiently high [S], Vmax is attained even in the presence of the inhibitor. However, competitive inhibitors increase the apparent Km for the given substrate. This means that in the presence of a competitive inhibitor, you need more substrate to achieve 1/2 Vmax. Examples of competitive inhibition include:
- the inhibition of succinic dehydrogenase by malonate and oxaloacetate
- the inhibition of alcohol dehydrogenase by ethanol in methanol poisoning
- the control of bacterial pathogens by sulpha drugs that compete with the substrate P-amino benzoic acid (PABA) for the active site of the enzyme
Non-competitive inhibition
Non-competitive inhibition takes place when the inhibitor and substrate bind at different sites of the enzyme. Here, the inhibitor can bind to either the free enzyme or the ES complex, preventing the reaction from taking place. The inhibitor has no structural resemblance to the substrate in this case.
Unlike competitive inhibitors, non-competitive inhibitors decrease the Vmax of the reaction. You can’t overcome non-competitive inhibition by increasing the [S]. Since these inhibitors don’t interfere with the binding of the substrate to the enzyme, the Km remains unaltered.
Heavy metal ions such as Ag+, Pb2+, and Hg2+ can non-competitively inhibit enzymes by binding to cysteinyl sulfhydryl groups.
Irreversible inhibition
Here, the inhibitor binds covalently to the enzyme and inactivates it in an irreversible manner. These inhibitors are usually toxic substances that poison enzymes. For example, iodoacetate is an irreversible inhibitor of certain enzymes such as papain and glyceraldehyde 3-phosphate dehydrogenase.
Iodoacetate combines with sulfhydryl (-SH) groups at the active sites of these enzymes and renders them inactive. Another good example of irreversible inhibition is cyanide, which can kill animals by inhibiting the enzyme cytochrome oxidase.
Suicide inhibition is a specialized form of irreversible inhibition where the original inhibitor (the structural analogue or competitive inhibitor) is converted to a more potent form by the same enzyme it was supposed to inhibit. The new inhibitor formed as a result binds irreversibly with the enzyme (in contrast to the original inhibitor that binds reversibly).
A good example of suicide inhibition is allopurinol, a drug that inhibits the enzyme xanthine oxidase. It is used in the treatment of gout.
Allosteric inhibition
Some enzymes possess additional sites besides the active site, known as allosteric sites. Such enzymes are known as allosteric enzymes. Certain substances referred to as allosteric modulators (effectors or modifiers) bind to the allosteric site and regulate the enzyme activity.
When a positive (+) allosteric effector binds to the allosteric site (known as the activator site), the enzyme activity is increased. On the other hand, a negative (-) allosteric effector can inhibit the enzyme activity by binding to the allosteric site (called inhibitor site in this case).
For example, glucose-6-phosphate is an allosteric inhibitor of the enzyme hexokinase. It plays an important role in feedback regulation during glycolysis.
Isoenzymes
Isoenzymes are multiple molecular forms of an enzyme, synthesized by different genes, occurring in the same organism, and having a similar substrate activity. More than a hundred known enzymes have been found to have isoenzymes. For example:
- Alpha-amylase of wheat endosperm has 16 isoenzymes.
- Lactic acid dehydrogenase has 5 isoenzymes.
- Alcohol dehydrogenase has 4 isoenzymes.
Isoenzymes are clinically significant because their levels in blood serum can help you diagnose tissue damage. For instance, different isoforms of lactate dehydrogenase (LDH) are elevated in heart, liver, or muscle injuries, making them valuable diagnostic markers.
Classification of enzymes
With years of dedicated effort, biochemists have discovered, isolated, and studied thousands of enzymes. Most of these enzymes have been classified into different groups based on the type of reactions they catalyse. Today, enzymes are divided into six different classes, with each class further being divided into 4-13 subclasses and named accordingly by a four-digit number, which you’ll learn about shortly.
Oxidoreductases/dehydrogenases
These are enzymes that catalyse simultaneous oxidation and reduction (oxidoreduction) reactions between two substrates S and S’ as shown below:
Sreduced + S’oxidised → Soxidised + S’reduced
Transferases
They are enzymes catalysing a transfer of a group G (other than hydrogen) between a pair of substrates S and S’ as seen below:
S – G + S’→ S + S’ – G
Examples of transferases include transaminases (transfer amino groups) and kinases (catalyse the phosphorylation of substrates by transferring phosphate groups, generally from ATP).
Hydrolases
They are enzymes that catalyse the hydrolysis of ester, ether, peptide, glycosidic, C-C, C-X or P-N bonds. They facilitate the breakdown of larger molecules into smaller molecules with the addition of water.
Examples of hydrolases include amylases, proteases, lipases, nucleases, maltase, invertase, and other digestive enzymes. They are abundantly found in lysosomes.
Lyases
These are enzymes that catalyze the cleavage of substrates into two parts without involving hydrolysis for the removal of groups. They leave behind a double bond at the place from where the group was removed.
Aldolase, carbonic anhydrase, and decarboxylase are examples of lyases.
Isomerases
This class includes all enzymes that catalyze the rearrangement of the molecular structure of the substrate to form isomers. They facilitate the interconversion of optical, geometric, or positional isomers. Isomerase is a classic example of this class.
Ligases
Finally, ligases are enzymes that catalyze covalent bonding of two substrates to form a large molecule. They facilitate the joining of bonds such as C-O, C-S, C-N, and P-O with the help of energy obtained from ATP.
Examples of ligases are phosphoenolpyruvate carboxylase, RUBP carboxylase, and DNA ligase.
Exoenzymes and endoenzymes
Most enzymes remain and function within the cell, and are known as intracellular enzymes or endoenzymes. Some of them are dissolved in the cytoplasmic matrix, while others are bound to particles such as mitochondria, ribosomes, and chloroplasts. For example, respiratory enzymes that are required to convert lactic acid to CO2 and water are present in the mitochondria.
However, certain enzymes leave the cell and function outside them. They are known as extracellular enzymes or exoenzymes. The main examples of such enzymes are digestive enzymes like salivary amylase, pepsin, and pancreatic lipase. It’s worth noting that enzymes retain their catalytic activity even after they are extracted from the cells. This property has given them many commercial applications in various industries.
Nomenclature of enzymes
In 1961, the International Union of Biochemistry (IUB) appointed an Enzyme Commission (EC) to devise some basic principles for the classification and nomenclature of enzymes. The IUB system has divided enzymes into six major classes that we’ve discussed above. Each of these classes is subdivided into many subclasses, which are further divided into sub-subclasses. Finally, a four-digit Enzyme Commission (EC) number is assigned to each enzyme.
In each number, the significance of each digit is as follows:
1st digit – Class
2nd digit – Subclass
3rd digit – Sub-subclass
4th digit – Individual enzyme
Co-factors
Like other proteins, enzymes are composed of one or many polypeptide chains. However, scientists have observed that in a lot of instances, certain non-protein constituents known as co-factors are bound to the enzyme in order to render it catalytically active. In these cases, the protein portion of the enzyme is known as the apoenzyme.
Biochemists have identified three different types of co-factors:
- Prosthetic groups
- Co-enzymes
- Metal ions
Prosthetic groups
Prosthetic groups are organic compounds that, unlike the other two co-factors, are tightly bound to the apoenzyme. For example, in peroxidase and catalase (enzymes that catalyze the breakdown of H2O2 to water and oxygen), haem is the prosthetic group and it constitutes a part of the active site of the enzyme.
Co-enzymes
Co-enzymes are organic compounds like prosthetic groups, but their association with the apoenzyme is only transient. It usually takes place during the course of catalysis. Moreover, co-enzymes serve as co-factors in several different enzyme-catalyzed reactions. The vital chemical components of many coenzymes are vitamins. For example, the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) contain the vitamin niacin.
Metal ions
A large number of enzymes require metal ions for their biological activity. These ions form coordination bonds with side chains at the active site, and simultaneously also form one or more coordination bonds with the substrate. For example, zinc is an important cofactor for the proteolytic enzyme carboxypeptidase.
If you remove the co-factor from the enzyme, the catalytic activity is lost. This proves that co-factors play a pivotal role in the catalytic activity of the enzyme.
Given below is a list of important metal ions and the metal-containing enzymes (metalloenzymes) they are found in.
| Metal ion(s) | Metalloenzymes |
|---|---|
| Fe2+, Fe3+ | Cytochrome oxidase, aconitase, catalase, peroxidase |
| Ca2+ | Succinate dehydrogenase, lipase |
| Mg2+ | Hexokinase, DNA polymerase, pyruvate kinase, phosphotransferase, enolase |
| Cu2+ | Cytochrome oxidase, tyrosinase |
| Co2+ | Peptidases, ascorbic acid oxidase |
| Mo | Nitrate reductase, dinitrogenase |
| Zn2+ | Carbonic anhydrase, Alcohol dehydrogenase, carboxypeptidase, LDH |
| Mn2+ | Arginase |
| Ni | Urease |
| Cl– | Salivary amylase |
What are enzymes and why are they important?
Enzymes are biological catalysts, mostly proteins, that speed up chemical reactions in living organisms without being consumed in the process. They are essential because virtually every metabolic reaction in your body, from digesting food to replicating DNA, depends on enzymes. Without them, these reactions would occur far too slowly to sustain life.
What is the difference between the lock and key model and the induced fit model?
The lock and key model (Emil Fischer, 1894) proposes that the enzyme’s active site has a rigid, pre-shaped structure that perfectly matches the substrate, much like a key fits a specific lock. The induced fit model (Koshland, 1958) suggests that the active site is flexible and changes its shape to accommodate the substrate upon binding, similar to how a glove molds around a hand. The induced fit model is more widely accepted today because it accounts for the flexibility of enzyme structures observed in experimental studies.
How do temperature and pH affect enzyme activity?
Every enzyme has an optimum temperature and pH at which it functions most efficiently. Below the optimum temperature, enzyme activity decreases because molecular collisions slow down. Above the optimum, the enzyme protein denatures and loses its three-dimensional shape permanently. Similarly, extreme pH values disrupt the ionic and hydrogen bonds that maintain the enzyme’s tertiary structure, reducing or eliminating its catalytic ability.
What is the difference between competitive and non-competitive inhibition?
In competitive inhibition, the inhibitor resembles the substrate structurally and competes for the same active site. You can overcome it by increasing the substrate concentration, and Vmax remains unchanged while apparent Km increases. In non-competitive inhibition, the inhibitor binds to a different site on the enzyme (not the active site), altering the enzyme’s shape and reducing its catalytic efficiency. Increasing substrate concentration does not reverse non-competitive inhibition, Vmax decreases, and Km stays the same.
What are co-factors and why do enzymes need them?
Co-factors are non-protein molecules that bind to enzymes and are required for their catalytic activity. They come in three types: prosthetic groups (tightly bound organic molecules like haem), co-enzymes (loosely bound organic molecules often derived from vitamins, such as NAD and NADP), and metal ions (such as Zn2+, Mg2+, and Fe2+). Without their respective co-factors, many enzymes cannot fold properly or catalyze reactions, and the protein portion alone (apoenzyme) remains inactive.
What is the Michaelis constant (Km) and what does it tell you?
The Michaelis constant (Km) is the substrate concentration at which an enzyme-catalyzed reaction proceeds at half of its maximum velocity (Vmax). It is a measure of the enzyme’s affinity for its substrate. A low Km value means the enzyme reaches half of Vmax at a low substrate concentration, indicating high affinity. A high Km value means more substrate is needed to reach half of Vmax, indicating lower affinity. Km is unique for each enzyme-substrate pair and is widely used to compare enzyme efficiency.
Shared this with my entire study group. The enzyme inhibition mechanisms section in particular cleared up a lot of confusion.
I teach introductory biology and often point my students to this resource. The explanations are accurate and accessible.
Thank you for including both the basic definitions and the deeper biochemistry. Covers everything from intro to advanced level.
I’m studying for my biology exam and this article saved me. Everything about enzymes is covered so clearly.
The real-world examples make enzymes so much more interesting. I actually enjoyed studying this chapter now.
Shared this with my entire study group. The enzyme inhibition mechanisms section in particular cleared up a lot of confusion.
This page on enzymes is now my primary reference for revision. Well-written, accurate, and free. Can’t ask for more.
As a medical student, understanding enzymes at the molecular level is crucial. This resource nails that balance between depth and clarity.
The real-world examples make enzymes so much more interesting. I actually enjoyed studying this chapter now.
As a medical student, understanding enzymes at the molecular level is crucial. This resource nails that balance between depth and clarity.
I teach introductory biology and often point my students to this resource. The explanations are accurate and accessible.
This is an excellent overview of enzymes. The level of detail is perfect for undergraduate students.